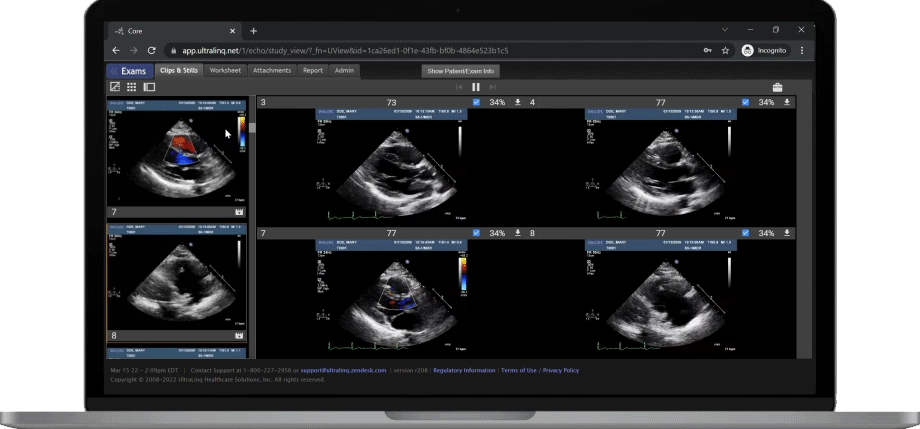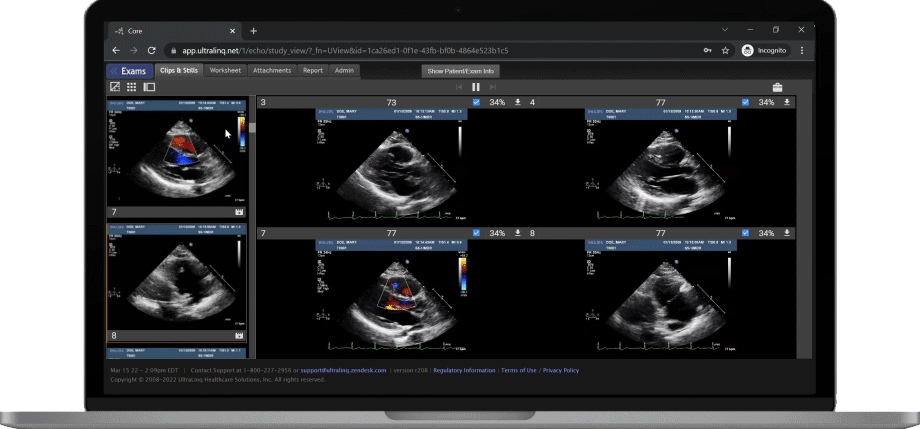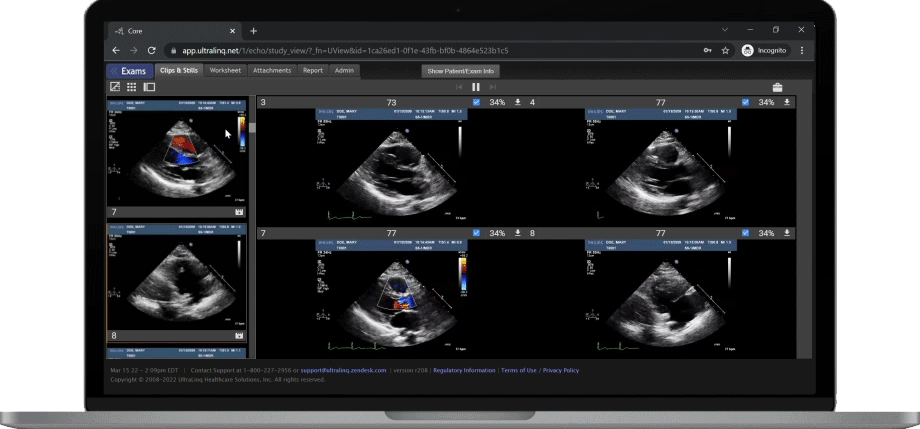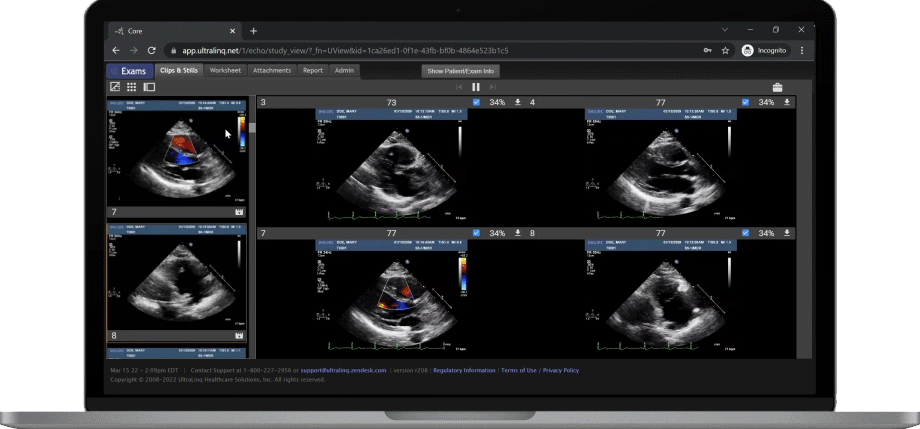


A Secure and Scalable Platform,
Trusted by Our Global Customers
Trusted by Our Global Customers
A Single Platform to Meet
Your Evolving Medical Practice
All your medical imaging and data requirements on a single, secure platform,
whether you are sole provider or multi-site medical group.
The UltraLinQ cloud-PACS Platform is designed to provide a secure, scalable, and compliant solution to meet the changing demands of healthcare delivery. Drive efficient patient diagnosis with DICOM images, archived patient reports or Holter Tests. We have everything you need to grow your patient services, all in one place.
Streamlined Reporting
Effortless multi-location exam access combined with fast upload, storage and retrieval
Secure and Scalable
A flexible and scalable solution with secure, industry-compliant patient data storage
Boost Workflow
Professional reporting designed to optimize productivity and clinical workflow
Cost Effective
A cloud-based solution shown to reduce the costs of image management and storage
Our PACS Specialties
UltraLinQ Holter Service
The new UltraLinQ Holter Service, designed to expand your cardiac monitoring service enabling clinicians to diagnose arrhythmias quickly and efficiently.
Low-cost Holter and
extended Holter Service
Latest 2-channel
ECG Biosensor Technology
Reports validated by certified
technicians, ready for Clinical
interpretation
In-clinic and at-home
clinical workflows
extended Holter Service
ECG Biosensor Technology
technicians, ready for Clinical
interpretation
clinical workflows

Save on Image
Management and Storage
We believe in transparency. We provide comprehensive solutions
to meet your requirements with no hidden fees.
Flexible pricing options to suit you - choose a pay-per-upload or subscription license
Access an extensive library of optimized medical worksheets
Unlimited cloud storage and number of users
Seamless integration with hospital systems
MFA and optional SSO capability
Free and automatic upgrades to platform
Unlimited technical support and training
No local server storage, maintenance or service costs
to meet your requirements with no hidden fees.
“I cannot imagine doing practice without this modality. I think having in my hand the ability to show the patient their study is a powerful tool. … It is a life changer for the doctors, as well as for the patients.”
Annie Varughese, MD, FACC
Chief Cardiologist
North Houston Family Medicine
read more testimonials
Ready to simplify
your workflow?
Schedule a demo
“I cannot imagine doing practice without this modality. I think having in my hand the ability to show the patient their study is a powerful tool. … It is a life changer for the doctors, as well as for the patients.”
Annie Varughese, MD, FACC







